EN 14476:2013+A2:2019 - Chemical disinfectants and antiseptics
Quantitative testing for the evaluation of virucidal activity in the medical field
Test method and requirements (Phase 2/Step 1)
Medical healthcare facilities, as well as institutions such as nurseries and nursing homes, are high-risk points for the spread of viral infection agents. For this reason, cleaning and disinfection in these areas become vital to prevent the outbreak of diseases, including the spread of hospital-acquired infections. Disinfectants and antiseptic products containing antiviral agents are widely used in the aforementioned sectors. EN 14476 is a quantitative test used to evaluate the virucidal action of such products.
The standard is implemented for products belonging to the following categories :
- Disinfection/hand washing
- Surface disinfection
- Disinfection of fabrics

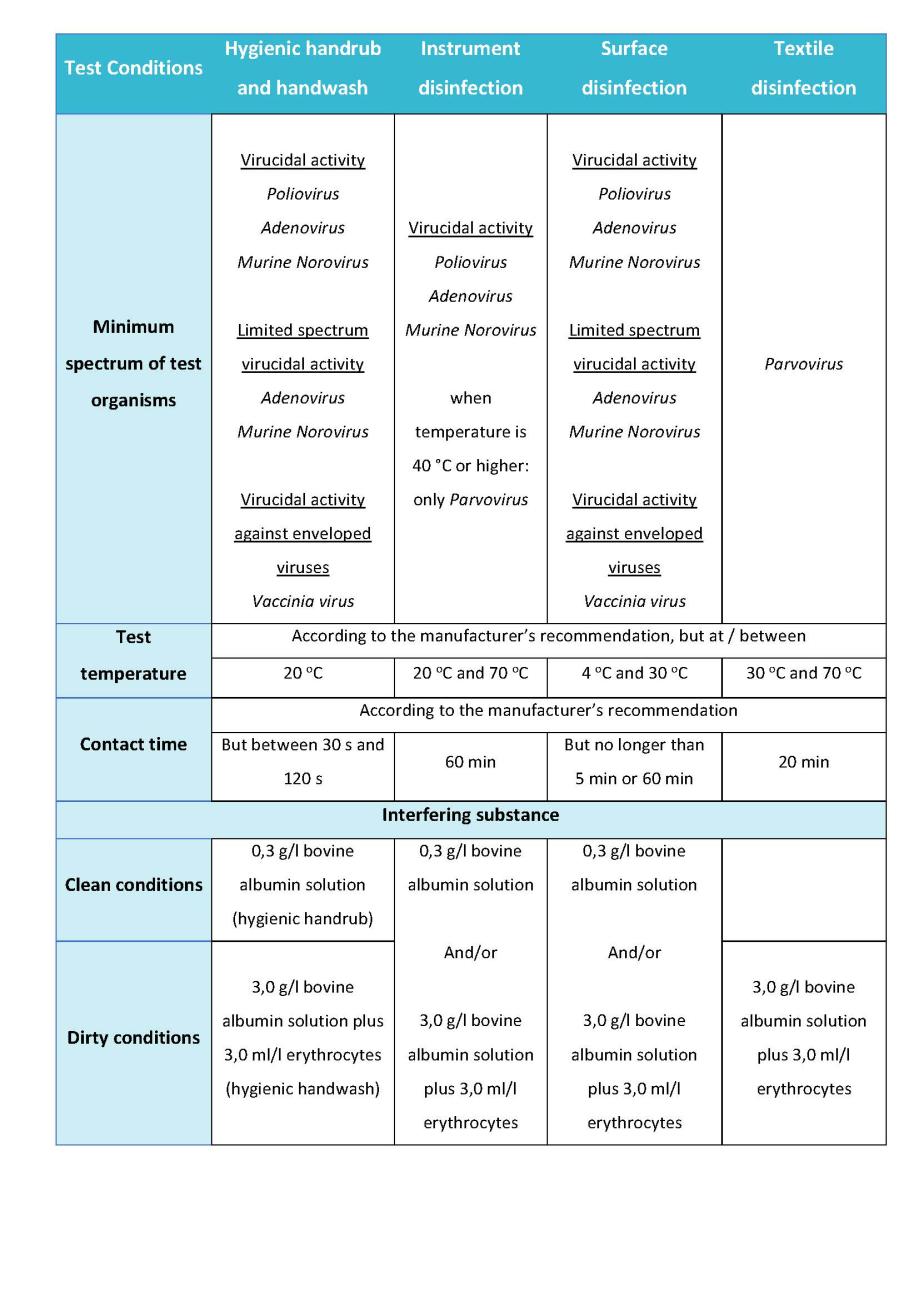
This standard specifies the minimum requirements for the virucidal activity of chemical disinfectants and antiseptic products.
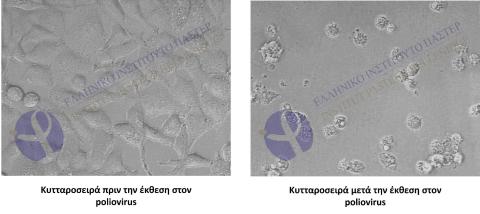
Method for testing virucidal activity
Subsequently, serial dilutions are performed, and live host cells are inoculated with them. If the tested product does not have antiviral activity against the specific virus, cell destruction is observed with the appearance of a cytopathic effect (CPE).
In recent years, the use of chemical disinfectants and antiseptic products is more relevant than ever, particularly due to the COVID-19 pandemic. The Diagnostic Department of the Hellenic Pasteur Institute was the first laboratory in Greece at the beginning of the pandemic to be on the front lines against the new SARS-CoV-2 and has since upgraded its infrastructure with a fully equipped biosafety level 3 (BSL3) laboratory.

At the Diagnostic Department of the National Institute of Hygiene and Hygiene, tests are carried out on the antiviral activity of disinfectant products according to the European standard EN 14476:2013+A2:2019 against the following viruses:
- Poliovirus
- Adenovirus
- Murine Norovirus
- Vaccinia virus
- SARS-CoV-2
- The results of the tests are sent within 30 days.
For more information about conducting antiviral tests, contact the laboratory at 210 647821 or by email s.makka@pasteur.gr/ myrto.koutantou@pasteur.gr.
