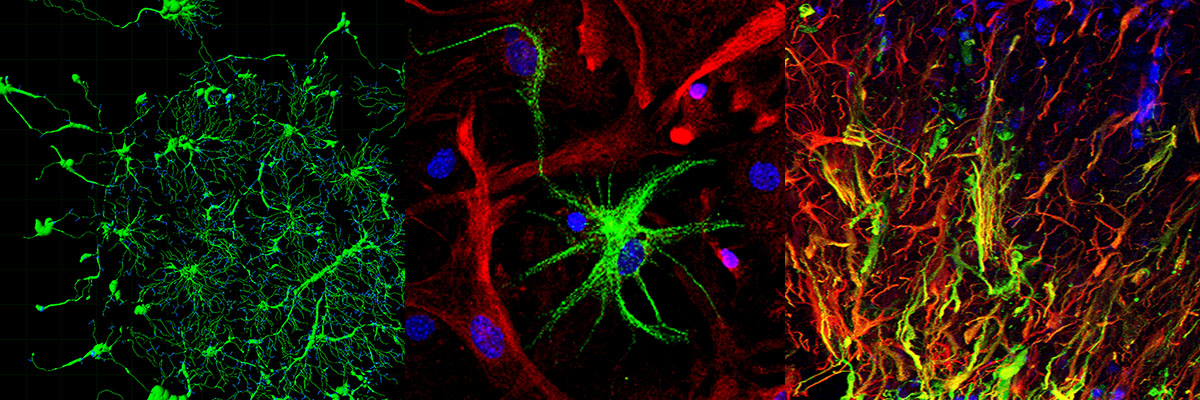

Mission of the group of Neural Stem Cells and Neuroimaging is the elucidation of the proliferation, differentiation and reprogramming properties of adult-derived Neural Stem Cells (NSCs) and parenchymal macroglia (astrocytes and microglia) in vitro and in vivo in animal models of brain lesion, neuroinflammation and neurodegenerative diseases. Our ultimate goal is to reveal the mode of action of new molecules in promoting neurogenesis and/or neurogenic reprogramming and restricting brain inflammation and use them to reduce neurodegeneration/ neuroinflammation.
The research of the group focuses on the investigation of the mechanism of action of novel genes, miRNAs and small molecules in 1) inducing direct neurogenic reprogramming of brain astrocytes in vitro and in vivo and 2) modifying microglia properties during Alzheimer’s Disease (AD) progression. To this end we are using a) primary mouse and human astrocytic cultures, b) recombinant viral vectors expressing proteins/ miRNAs of interest and c) inducible transgenic mice with cell type-specific and time-regulated protein expression or down-regulation to:
- Explore the regenerative potential and reveal the mechanism of action of neurogenic genes and neurogenesis-modulating miRNAs in driving brain astrocytes direct reprogramming to induced-neurons: in vitro applying molecular phenotype, functional and NGS analysis and in vivo, by astrocyte-specific forced expression of neurogenic molecules into the injured mouse brain.
- Follow, by whole animal 2-photon (2P) imaging, NVU dynamics in real time following brain injury and inflammation. To this end our group has established in the Institute’s Light Microscopy Unit (LMU) 2P intravital imaging of the CNS.
- Study the role of the late-onset AD risk factor BIN1 on microglia activation and its contribution in AD progression, by real time mouse brain intravital 2P-imaging to monitor dynamic microglia properties and interactions and by single cell NGS analysis to reveal activated microglia states and non-cell autonomous functions in the absence of microglial BIN1.
- Study the effect of chemical brain lesion on adult neurogenesis occurring in neurogenic and non-neurogenic brain areas by performing in vivo lineage tracing to elucidate the origin and final fate of newborn neurons.
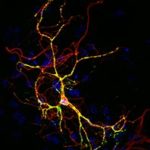
Generation of neurons by in vitro direct reprogramming of mouse and human cortical astrocytes and embryonic fibroblasts
Direct lineage reprogramming has emerged as a promising, fast approach for manipulating cell fate to obtain customized functional neuronal cell types. A step further, utilization of endogenous brain cells that can be directly reprogrammed into neurons, remains a challenge with important clinical applications for the treatment of neurodegeneration.
Recent studies demonstrate that various cell types, including brain astrocytes and skin fibroblasts, can be reprogrammed to neural precursor cells (NPCs) and neurons via challenging certain genes levels. Our aim is to investigate whether in lineage distant somatic cell types residing inside and outside the CNS can be turned into NPCs and neurons upon forced expression of factors that induce neurogenesis during development. To this end we have demonstrated that: a) the neurogenic molecule CEND1 can reprogram mouse astrocytes towards GABA+ neurons and acts synergistically with NEUROG2 to trans-differentiate them towards proliferating NPCs and subtype-specific neurons, b) CEND1 and NEUROG2 possess a broader neurogenic potential, inducing neuronal reprogramming of MEFs , c) CEND1 is a key downstream player in NEUROG2-induced astrocytic reprogramming participating in a positive feedback loop leading to neurogenesis and d) Double over-expression of CEND1 and NEUROG2 results in activation of Wnt/ beta-catenin signaling pathway driving astrocytes to acquire NSC multipotent character. Taken together, our results demonstrate that CEND1 is not only a potent inducer of neuronal differentiation in neural precursor cells as previously shown (Katsimpardi et al., 2008), but has a neurogenic capacity in different cell contexts. Currently we are expanding our studies to exploiting the mechanisms of direct cell reprogramming of human primary astrocytes to functional neurons through neurogenic molecules forced expression.
Members involved
Katerina Aravantinou-Fatorou, Seyyedeh Vejdani
Relevant publications
Aravantinou-Fatorou K, Thomaidou D (2020). In vitro direct reprogramming of mouse and
human astrocytes to induced neurons. In “Stem Cells and Tissue Repair: Methods and
Protocols”, 2nd edition, Springer Science Methods Mol Biol. 2155:41-61. doi: 10.1007/978-1-
0716-0655-1_4.
Aravantinou-Fatorou K, Ortega, F, Chroni-Tzartou D., Antoniou, Poulopoulou C., N., Politis
P.K., Berninger B. Matsas R and Thomaidou D. (2015) Cend1 and Neurogenin2 reprogram
mouse astrocytes and embryonic fibroblasts to induced neural precursor cells and
differentiated neurons, Stem Cell Reports; 5(3): 405-18 doi: 10.1016/j.stemcr.2015.07.012.
Aravantinou-Fatorou K, Vedjani S, Thomaidou D. Cend1 and Neurog2 efficiently reprogram human cortical astrocytes to induced-neurons (2021). Int J Dev Biol. (2021) Online ahead of print. doi: 10.1387/ijdb.210148dt.
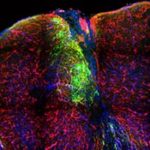
Mechanisms of direct reprogramming of reactive astrocytes to differentiated neurons in vitro and in vivo following forced expression of neurogenic miRNAs
Our group is interested in studying the molecular mechanisms governing direct lineage reprogramming to induced neurons (iNs), as well as, exploiting the mode of action of neurogenic miRNAs in promoting neuronal trans-differentiation of somatic cell types. miRNAs have emerged as critical post-transcriptional modulators of gene expression by simultaneously targeting multiple genes, thus they appear as attractive candidates to regulate direct cell reprogramming through fine-tuning the expression levels of neurogenic factors. Our efforts are focusing towards the identification of miRNAs that are potent to induce direct cell reprogramming of primary astrocytes to induced-neurons (iNs) in vitro and in vivo and elucidation of their mechanisms of action at the transcriptional and post-transcriptional level. To this end we are using neurogenic miRNA-mimics and recombinant viral vectors expressing selected neurogenic miRNAs along with fluorescent proteins to: a) in vitro explore the coding and non-coding transcriptome profile of reprogrammed astrocytes with next generation sequencing and functional assays and b) assess the capacity of certain neurogenic miRNAs to instruct direct neuronal reprogramming of astrocytes following their in vivo forced expression in animal models of brain trauma.
Members involved
Elsa Papadimitriou, Pari Koutsoudaki, Irini Thanou, Mary Margariti
Relevant publications
Papadimitriou E, Koutsoudaki PN, Thanou I, Karagkouni D, Karamitros T, Chroni-Tzartou D, Gaitanou M, Gkemisis C, Margariti M, Xingi E, Tzartos SJ, Hatzigeorgiou A, Thomaidou D (2023). A miR-124-mediated post-transcriptional mechanism controlling the cell fate switch of astrocytes to induced-neurons. Stem Cell Reports, 11;18(4):915-935 doi: 10.1016/j.stemcr.2023.02.009
Papadimitriou, E; Thomaidou, D (2024) Post-transcriptional mechanisms controlling neurogenesis and direct neuronal reprogramming: 19(9):p 1929-1939, doi: 10.4103/1673-5374.390976.
.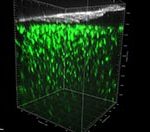
An open window into the brain through intravital 2-photon imaging: Study of Neurovascular Unit (NVU) interactions and astroglia-microglia dynamics in brain inflammation and disease
The term “Neurovascular Unit” (NVU) is used to describe the integrated system of vascular and neuronal cells and the surrounding glia working in concert to enable proper brain homeostasis and function. The NVU is affected in many CNS conditions and plays a role in their pathology thus study of the cellular interactions in the pathological NVU could contribute to the identification of numerous potential targets for treatment in the context of CNS neurodegeneration/ neuroinflammation. The dynamics of such processes can be adequately captured by in vivo 2-photon imaging, which allows the study of cellular responses to environmental stimuli and cell-cell interactions in the living brain in real time. Accordingly, to explore the dynamic interactions of the NVU following acute and chronic brain injury, we are monitoring by intravital 2-photon imaging the morphological changes of perivascular astrocytes, as well as their dynamic interactions with perivascular microglia and blood vessels under physiological conditions and during LPS-induced systemic inflammation or laser-induced acute brain injury, using transgenic mice expressing fluorescent proteins under astroglia-, microglia- and pericyte-specific promoters. In a parallel project, led by Dr. Vasso Kyrargiri, microglia-specific transgenic mice are used to visualize by intravital 2-photon imaging microglia dynamics in a mouse model of demyelination.
Members involved
Pari Koutsoudaki, Evangelia Xingi (Light Microscopy Unit)[f1] , Irini Thanou, Mary Margariti
Collaborations with: Prof. Frank Kirchhoff, Univ. of Saarland; Dr. Jean-Yves Tinevez, Institut Pasteur Dr. Vassiliki Kyrargiri, Hellenic Pasteur Institute
Roufagalas I, Avloniti M, Fortosi A, Xingi E, Thomaidou D, Probert L, Kyrargyri V. Novel cell-based analysis reveals region-dependent changes in microglial dynamics in grey matter in a cuprizone model of demyelination. Neurobiol Dis. (2021) Online ahead of print doi: 10.1016/j.nbd.2021.105449.
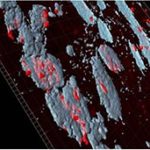
Chemobrain: Effect of chemotherapy on adult neurogenesis occurring in neurogenic and non-neurogenic brain areas
Adult mammalian brain neural stem/progenitor cells have a spatially restricted distribution and neurogenesis takes place only in specific brain areas. However recently, it has been proposed that a low level of neurogenesis also occurs in “non-neurogenic regions”, such as the striatum and to a lesser extent the cortex. Importantly, brain lesions have succeeded to unmask neurogenic activity in such regions in laboratory rodents. To this end we are investigating whether disturbance of the adult mouse SVZ, through stereotaxic intraventricular injections of mitotoxic/chemotherapeutic agents activates cells of neighboring non-neurogenic regions to acquire a neural progenitor potential. Our current data support the hypothesis that administration of mitotoxic/chemotherapeutic agents in the cerebrospinal fluid (CSF) is responsible for SVZ disruption and ectopic presence of newborn neurons in non-neurogenic areas. The fact that several mitotoxic factors are widely used as chemotherapeutic agents and that patients undergoing chemotherapy exhibit progressive cognitive deficits, raises the question whether these treatments lead to disruption of neurogenesis. Therefore, further study of this SVZ-disorganization model will shed light into important questions related to NPCs and parenchymal brain cells’ response to chemotherapeutic treatment.
Members involved
Irini Thanou, Mirka Fourmouzi, Eleni Makarouni, Pari Koutsoudaki
Collaborations with: Dr. Federico Luzzati, University of Turin; Prof. Vassilis Gorgoulis, Medical School, University of Athens
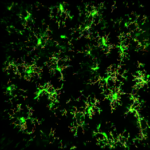
Microglia dynamics and response to neuroinflammation in the absence of AD risk factor BIN1
A number of recent studies highlight the contribution of microglial cells in AD progression, rendering them a potential cell target to combat the disease. BIN1 is the second most important sporadic AD risk factor with high expression levels in microglia, however the contribution of BIN1 different expression levels in microglial cells in response to neuroinflammation and AD pathogenesis and both in humans and animal models has not been investigated yet. In this project we use transgenic mice harboring fluorescent microglia cells not expressing BIN1, to investigate by single cell transcriptomics and live cell imaging microglial cells distinct activation signatures and states, as well as, their impact on the other glial and neuronal cell types.
Members involved
Mary Margariti, Elsa Papadimitriou, Irini Thanou
Collaboration with Dr. Marcos Costa, Institut Pasteur Lille & Dr. Jean-Yves Tinevez, Institut Pasteur Paris
Public Investments’ Program 2023-2025: National Research Network: Genetic basis of Alzheimer’s and Parkinson’s Diseases on the basis of Precision Medicine, (PI).
Program Transversal de Resérche (PTR) Grant 2021-2022: Microglia Imaging in Alzheimer’s Disease (MIAD), in collaboration with Dr. M. Costa, Institut Pasteur Lille and Dr. J.-Y. Tinevez, Institut Pasteur Paris (PI).
Public Investments’ Program 2020-2022: National Research Network: Research in Neurodegenerative Diseases Based on the basis of Precision Medicine (PI).
EU Horizon 2020 COST Action 2018-2022: Correlated Multimodal Imaging – COMULIS CA17121 (PI)
Fondation Santé – 2017-2018: “Elucidation of miRNA-mediated transcription and epigenetic mechanisms controlling trans-differentiation of astrocytes to induced neurons” (PI).
IKY Post-doc fellowship to Dr K. Aravantinou 2017-2018: "Direct reprogramming of human astrocytes to induced neurons" (PI).
Ministry of Education Infrastructure 2014-2020 Grant BIOIMAGING-GR 2017-2021: BioImaging-GR: A Greek Research Infrastructure for Visualizing and Monitoring Fundamental Biological Processes (PI).
Stavros Niarchos Foundation Grant 2016-2021: Development of innovative biological products and services for infectious and neurodegenerative diseases, Coordinated by R. Matsas & V. Miriagou (PI).
Greek Ministry of Education 2012-2015: EXCELLENCE II GRANT 3713 –ASTRO-TRACE: Astroglia activation and reprogramming in brain injury and repair: an open window into the brain through intravital imaging (PI).
IKYDA-2014 Greek German Grant, 2014-2015: Astroglia activation and reprogramming in brain injury and repair: analysis by in vivo imaging (2P-LSM) of the brain (PI).
General Secretariat for Research and Technology - European Social Fund (ESF) and the Greek State, Action “KRIPIS” of the Operational Strategic Reference Framework, NSFR 2007-2013 GRANT InfeNeuTra - MIS450598: Infectious and neurodegenerative diseases in the 21st century: study of basic mechanisms for the development of the translational research and cutting edge technologies aiming to effective diagnosis prevention and therapy, Coordinated by K. Karagouni (PI).
Institut Pasteur International Network and Carnot Foundation 2013 Grant for organization of RIIP Regional Course: Digital image processing/analysis tools in Light Microscopy: From the basics and beyond, Organized by H. Boleti, D. Thomaidou, J.C. Olivo -Marin.
EPEAK Archimidis Grant HEATSTEMAB, 2012-2014: Study of Heat Shock Protein 90 in Cancer Stem Cells properties, Coordinated by E. Patsavoudi (collaborating PI).
EU FP7 Program Grant 264083 NEUROSIGN 2010-2013: Development of a Centre of Excellence in Neurosignalling, Coordinated by S. Tzartos - (WP leader responsible for Infrastructure Acquisition and In Vivo Imaging).
Institut Pasteur Paris Grand Program Horizontal (GPH), 2004-2008. Stem Cells: Control of Stem Cells Neurogenesis in the Adult Brain, Coordinated by J.M. Heard (PI).
Greek General Secretariat of Research and Technology Human Networks of Scientific and Technological Training Program, 2004-2005. Applications of light microscopy in Biomedical Research and Diagnosis (co-PI with H. Boleti).
Greek General Secretariat of Research and Technology (GSRT) and British Council Program of Greek-English Collaboration. 2005-2007 Elucidation of the function of the neurogenic gene bm88 in a mouse knock-out model (PI).
Greek General Secretariat for Research and Technology Programme EPAN, 2004-2006: Neural Stem Cell Therapies for Neurodegenerative Diseases: Determination of a “molecular signature” for neuronal fate, YB26, Coordinated by R. Matsas (PI).
Dimitra Thomaidou has the following record in educational activities :
|
2017-2024 |
Teaching in Athens International Master’s Programme in Neurosciences, part of the Network of European Neuroscience Schools (NENS), coordinated by Department of Biology, University of Athens |
|
2005-2017 |
Teaching in Inter-Departmental MSc Programme: Applications of Biology in Medicine, Department of Biology and School of Health Sciences, University of Athens |
|
2004-2024 |
Teaching in Inter-Departmental MSc Programme: Clinical Biochemistry-Molecular Diagnosis, Departments of Biology and Chemistry, University of Athens |
|
2015-2024 |
Teaching in Inter-Departmental MSc Programme: From Bench to Bedside, School of Health Sciences, Department of Medicine, Department of Molecular Biology and Genetics of Democritus University of Thrace |
|
2010-2024 |
Teaching in MSc Program: Advanced systems and methodologies in Biomedical Technology, Biomedical Engineering Department of the University of West Attica |
|
2005-2024 |
Training of numerous under-graduate and post-graduate students of HPI and other Greek Universities/Research Institutes in Confocal & 2-Photon Microscopy and Live Cell Imaging technologies. |
The following workshops have been organized or co-organized by our group :
|
2019 |
Advanced hands-on ImageJ/Fiji macro workshop, supported by Bioimaging-GR, 5-11 May 2019, HPI Athens. |
|
2018 |
Live Cell Imaging Workshop “Revealing Life’s Hidden Secrets through New technology””, co-organized by Light Microscopy Unit and LEICA-Microsystems, supported by Bioimaging-GR, 5-6 June 2018, HPI Athens. |
|
2013 |
International Digital image processing/analysis tools in Light Microscopy: From the basics and beyond”, supported by the Institut Pasteur International Network (RIIP) and the Carnot Foundation (2013) HPI, Athens. |
|
2013 |
EU-REGPOT ‘NeuroSign’ European training workshop on: Live Cell Imaging and Electrophysiology, HPI, Athens. |
|
2012 |
EU-REGPOT ‘NeuroSign’ European training workshop on: Animal models of neurodegeneration and assessment of motor and cognitive function, HPI, Athens. |
|
2004-2005 |
Workshops on Modern light microscopy techniques in biomedical research" HPI, Athens. |
|
2003 |
FENS/IBRO Winter School: NEURAL STEM CELLS from specification and nervous system patterning to therapies for neurodegenerative diseases, Kitzbuhel, Austria. |
Communicating science to the public is an important for all members of our group. To this end we are participating in several dissemination activities including :
- European Researchers’ Night
- Athens Science Festival
- Brain Awareness Week (public events organized by the Hellenic Society for Neurosciences and the Dana Alliance for Brain Research)
- Lectures to high school students organized in HPI
2024
Papadimitriou, E; Thomaidou, D (2024) Post-transcriptional mechanisms controlling neurogenesis and direct neuronal reprogramming: 19(9):p 1929-1939, doi: 10.4103/1673-5374.390976.
2023
Papadimitriou E, Koutsoudaki PN, Thanou I, Karagkouni D, Karamitros T, Chroni-Tzartou D, Gaitanou M, Gkemisis C, Margariti M, Xingi E, Tzartos SJ, Hatzigeorgiou A, Thomaidou D (2023). A miR-124-mediated post-transcriptional mechanism controlling the cell fate switch of astrocytes to induced-neurons. Stem Cell Reports, 11;18(4):915-935 doi: 10.1016/j.stemcr.2023.02.009.
Xingi E, Koutsoudaki P, Thanou I, Phan M.S, Margariti M, Scheller A, Tinevez J-Y, Kirchhoff F, Thomaidou D (2023). LPS-induced systemic inflammation affects the dynamic interactions of astrocytes and microglia with the vasculature of the mouse brain cortex Cells 12(10), 1418 doi.org/10.3390/cells12101418.
2022
Aravantinou-Fatorou K, Vejdani S, Thomaidou D. (2022) Cend1 and Neurog2 efficiently reprogram human cortical astrocytes to neural precursor cells and induced-neurons. Int J Dev Biol. 66(1-2-3):199-209. doi: 10.1387/ijdb.210148dt.
2021
Roufagalas I, Avloniti M, Fortosi A, Xingi E, Thomaidou D, Probert L, Kyrargyri V. Novel cell-based analysis reveals region-dependent changes in microglial dynamics in grey matter in a cuprizone model of demyelination. Neurobiol Dis. (2021) Online ahead of print doi: 10.1016/j.nbd.2021.105449.
2020
Aravantinou-Fatorou K, Thomaidou D (2020). In vitro direct reprogramming of mouse and human astrocytes to induced neurons. Methods Mol Biol. 2155:41-61. doi: 10.1007/978-1-0716-0655-1_4.
Fotopoulou E., Lykogianni M, Papadimitriou E, Mavrikou S, Machera K, Kintzios S, Thomaidou D*, Aliferis KA*. (2020) *equal correspondence. Mining the effect of the neonicotinoids imidacloprid and clothianidin on the chemical homeostasis and energy equilibrium of primary mouse neural stem/progenitor cells using metabolomics Pesticide Biochemistry and Physiology, doi:org/10.1016/j.pestbp.2020.104617.
2019
Koutmani Y, Gampierakis IA, Ximerakis M, Koutsoudaki PN, Polissidis A, Polyzos A, Agrogiannis G, Karaliota, Thomaidou D., Rubin L., Politis PK, Karalis KP. (2019) CRH promotes the neurogenic activity of adult neural stem cells via BMP4 suppression, Cell Reports 29(4):932-945. doi: 10.1016/j.celrep.2019.09.037
Tzortzopoulos A, Thomaidou D, Gaitanou M, Matsas R and Skoulakis E. (2019) Expression of mammalian BM88/CEND1 in Drosophila affects nervous system development by interfering with precursor cell formation. Neurosci Bull. 5(6):979-995. doi: 10.1007/s12264-019-00386-5.
Zoi I, Karamouzis MV, Xingi E, Sarantis P, Thomaidou D, Lembessis P, Theocharis S, Papavassiliou AG. Combining RANK/RANKL and ERBB-2 targeting as a novel strategy in ERBB-2-positive breast carcinomas. Breast Cancer Res. (2019) 21(1):132. doi: 10.1186/s13058-019-1226-9.
Anestis, A; Sarantis P, Theocharis S, Zoi I; Tryfonopoulos, D, Korogiannos, A Koumarianou, A, Xingi E, Thomaidou D, Kontos M, Papavassiliou, AG; Karamouzis, MV (2019) Estrogen Receptor beta increases sensitivity to enzalutamide in Androgen receptor-positive Triple-Negative Breast Cancer. J Cancer Res Clin Oncol. 145(5):1221-1233. doi: 10.1007/s00432-019-02872-9.
2018
Sassone J, Papadimitriou E, Thomaidou D (2018) Regenerative Approaches in Huntington's Disease: From Mechanistic Insights to Therapeutic Protocols Front Neurosci. 2;12:800. doi: 10.3389/fnins.2018.00800.
2017
Pallaki P, Papadimitriou E, Georganta E.M, Serafimidis I, Papakonstantinou M.P, Agalou A, Koutloglou S, Symeonof A, Tserga A, Papanikolaou V, Thomaidou D, Gaitanou M. and Georgoussi Z. (2017) A novel regulatory role of RGS4 in STAT5B activation and its effect on neural precursors proliferation and neurite outgrowth Neuropharmacology; 117:408-421. doi: 10.1016/j.neuropharm.2017.02.012
2016
Stivarou T, Stellas D, Vartzi G, Thomaidou D and Patsavoudi E (2016) MAb 4C5 targeting eHSP90, inhibits breast cancer stem cell derived tumor growth in vitro and in vivo. Cancer Biol. Ther.; 2; 17(8):799-812. doi: 10.1080/15384047.2016.1195041.
2015
Aravantinou-Fatorou K, Ortega, F, Chroni-Tzartou D., Antoniou, Poulopoulou C., N., Politis P.K., Berninger B. Matsas R and Thomaidou D. (2015) Cend1 and Neurogenin2 reprogram mouse astrocytes and embryonic fibroblasts to induced neural precursor cells and differentiated neurons, Stem Cell Reports; 5(3): 405-18. doi: 10.1016/j.stemcr.2015.07.012.
Thomaidou D and Patsavoudi E. (2015) Extracellular HSP90 in cancer invasion and metastasis: from translational research to clinical prospects, J. Analyt. Oncol. (Invited Review) 4(4):178-190.
2014
Thomaidou D. (2014) Neural Stem Cells transplantation in an animal model of traumatic brain injury; Methods Mol. Biol., 1210: 9-21. doi: 10.1007/978-1-4939-1435-7_2.
2012
Ziavra D., Makri G, Giompres P., Taraviras S., Thomaidou D, Matsas R, Mitsacos A, Kouvelas ED (2012). “Embryonic Stem Cells transplanted in the striatum of a mouse model of Parkinson’s disease differentiate to neuronal phenotypes and reduce rotational deficit CNS Neurol. Disord. Drug Targets. 11(7):829-35. doi: 10.2174/1871527311201070829.
2011
Elkouris M, Balaskas N, Poulou M, Politis PK, Panayiotou E, Malas S, Thomaidou D, Remboutsika E (2011). Sox1 maintains the undifferentiated state of cortical neural progenitor cells via the suppression of Prox1-mediated cell cycle exit and neurogenesis. Stem Cells 29: 89-98. doi: 10.2174/1871527311201070829.
2010
Lavdas AΑ., Chen J., Papastefanaki F., Schachner M. Matsas R., and Thomaidou D. (2010). Transplantation of Schwann cells over-expressing L1 enhance functional recovery in a mouse spinal cord compression model Exp. Neurol. 221:206-216. doi: 10.1016/j.expneurol.2009.10.024
Makri G., Lavdas A., Katsimpardi L., Thomaidou D.* and Matsas R.* (2010). *Joint senior authors. Transplantation of embryonic neural stem/ precursor cells overexpressing BM88/Cend1 enhances the generation of neuronal cells in the injured mouse cortex. Stem Cells, 28: 127-139. doi: 10.1016/j.expneurol.2009.10.024
2008
Katsimpardi L., Gaitanou M., Malnou C., Charneau P., Lledo PM., Matsas R. and Thomaidou D. (2008) BM88/Cend1 expression levels are critical for proliferation and differentiation of subventricular zone-derived neural precursor cells. Stem Cells, 26: 1796-807. doi: 10.1634/stemcells.2007.
Politis PK, Thomaidou D, Matsas R. (2008) Coordination of cell cycle exit and differentiation of neuronal progenitors. Cell Cycle. Mar 15;7(6):691-7. doi: 10.4161/cc.7.6.5550.
2007
Politis PK, Thomaidou D, Matsas R. (2008) Coordination of cell cycle exit and differentiation of neuronal progenitors. Cell Cycle. Mar 15;7(6):691-7. doi: 10.4161/cc.7.6.5550.
Politis PK, Makri G, Thomaidou D, Geissen M, Rohrer H, Matsas R. (2007) BM88/CEND1 coordinates cell cycle exit and differentiation of neuronal precursors. Proc Natl Acad Sci US A, 104: 17861-6. doi: 10.1073/pnas.0610973104.
Papastefanaki, F., Chen J, Lavdas A, Thomaidou D, Schachner M and Matsas R (2007) Grafts of Schwann cells engineered to express PSA-NCAM promote functional recovery after spinal cord injury, Brain, 130: 2159-74. doi: 10.1093/brain/awm155.
2006
Georgopoulou, N., Hurel, C., Politis P., Gaitanou, M Matsas R. and Thomaidou D. (2006) BM88 is a dual function molecule inducing cell cycle exit and neuronal differentiation of neuroblastoma cells via cyclin D1 down-regulation and pRb hypophosphorylation JBC, 281: 33606-33620. doi: 10.1074/jbc.M602689200.
2004
Koutmani Y., Hurel, C. Patsavoudi E., Haeck M., Gotz M., Thomaidou D.* and Matsas R. * *equal contribution. (2004) “BM88 is a marker of proliferating neuroblasts that will differentiate into the neuronal lineage”, Eur. J. Neurosci. 20:2509-23. doi: 10.1111/j.1460-9568.2004.03724.x
Full publication list at : https://www.ncbi.nlm.nih.gov/pubmed/?term=Thomaidou+D
CΗAPTERS IN BOOKS
Aravantinou-Fatorou K, Thomaidou D (2020). In vitro direct reprogramming of mouse and human astrocytes to induced neurons. In “Stem Cells and Tissue Repair: Methods and Protocols”, 2nd edition, Springer Science Methods Mol Biol. 2155:41-61. doi: 10.1007/978-1-0716-0655-1_4.
Thomaidou D. (2014) Neural Stem Cells transplantation in an animal model of traumatic brain injury, in “Stem Cell and Tissue Repair: Methods and Protocols”. Published by Springer, Chryssa Kioussi editor (Chapter 2).
Thomaidou D., Politis PK., and Matsas R. (2010) Neurogenesis in the Central Nervous System: Cell cycle progression / exit and differentiation of neuronal progenitors, in “Cell Cycle Regulation and Differentiation in Cardiovascular and Neural Systems”, published by Springer Verlag., Antonio Giordano and Umberto Galderisi editors (Chapter 8).



